ISO 13485:2016
Quality Management System For Medical Device
Description:
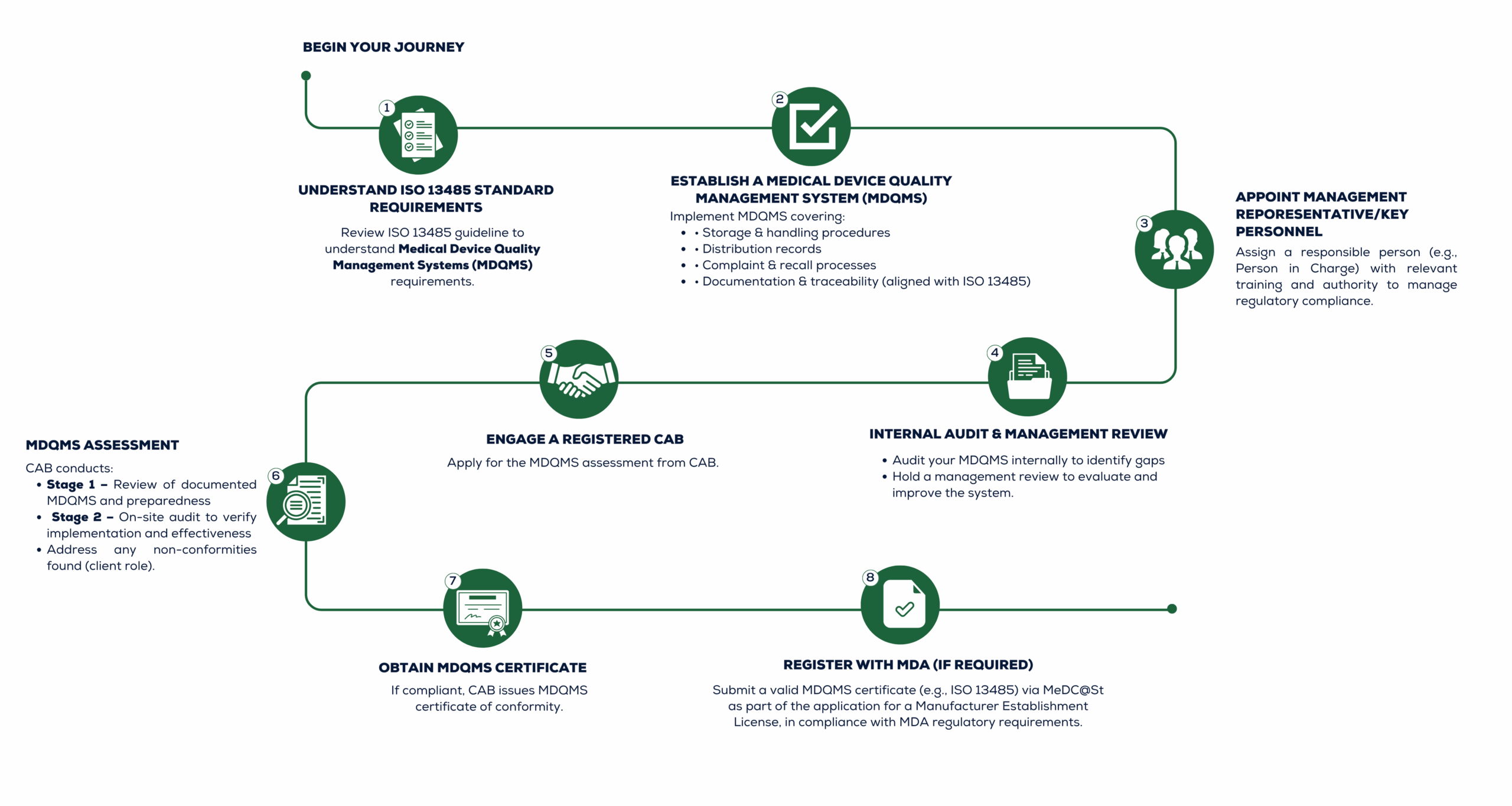
ISO 13485 is an internationally recognized standard for the quality management system (QMS) specifically designed for the medical device industry. This standard outlines the requirements for establishing, implementing, and maintaining a quality management system that ensures the consistent design, development, production, and distribution of medical devices while adhering to regulatory requirements and maintaining patient safety. ISO 13485 certification is specific to the medical device industry and differs from other ISO standards, such as ISO 9001, which is a broader quality management standard applicable to various industries.
Main Components:
Quality Management System:
ISO 13485 specifies the requirements for an organization’s quality management system. This system should address various aspects of the product life cycle, including design, development, manufacturing, distribution, and post-market activities.
Risk Management:
The standard emphasizes the need for risk management throughout the entire product lifecycle. This involves identifying and assessing risks associated with medical devices and implementing measures to mitigate these risks.
Regulatory Compliance:
ISO 13485 helps organizations comply with regulatory requirements specific to the medical device industry. It aligns with various global regulations, including the European Medical Device Regulation (MDR) and the US Food and Drug Administration (FDA) regulations.
Documentation and Record Keeping:
The standard requires organizations to maintain detailed documentation of processes, procedures, and records related to the development, production, and distribution of medical devices. This documentation is crucial for traceability and accountability.
Supplier Management:
The standard also requires effective management of suppliers and external partners to ensure that the materials and components used in medical devices meet appropriate quality standards.
Who should be certified:
Benefits:
- Enhanced Quality: ISO 13485 provides a framework for implementing a robust quality management system that helps organizations ensure consistent product quality and safety. This can lead to fewer defects, reduced product recalls, and improved patient outcomes.
- Global Market Access: Many countries and regions require medical device manufacturers to comply with specific regulatory standards. ISO 13485 certification is widely recognized internationally and can serve as evidence of your commitment to quality and regulatory compliance, facilitating market access in various countries.
- Regulatory Compliance: The standard is designed to align with regulatory requirements in the medical device industry.
- Competitive Advantage: ISO 13485 certification can set your organization apart from competitors that are not certified. It demonstrates your dedication to quality and safety, which can be a strong selling point when attracting customers and partners.
- Risk Management: ISO 13485 places a significant emphasis on risk management throughout the product lifecycle. This helps organizations identify potential risks and implement measures to mitigate them, leading to safer products and reduced liability.
- Improved Efficiency: Implementing the processes and procedures outlined in ISO 13485 can lead to increased operational efficiency. By streamlining workflows, reducing errors, and optimizing processes, organizations can save time and resources.
- Customer Confidence: Certification indicates to your customers that your products meet high standards of quality and safety. This can foster trust and confidence in your brand, leading to increased customer satisfaction and loyalty.